Healthcare
Logistics
- GDP-Certified
- Cold Chain Management
- Freight Forwarding
- Customs Clearance
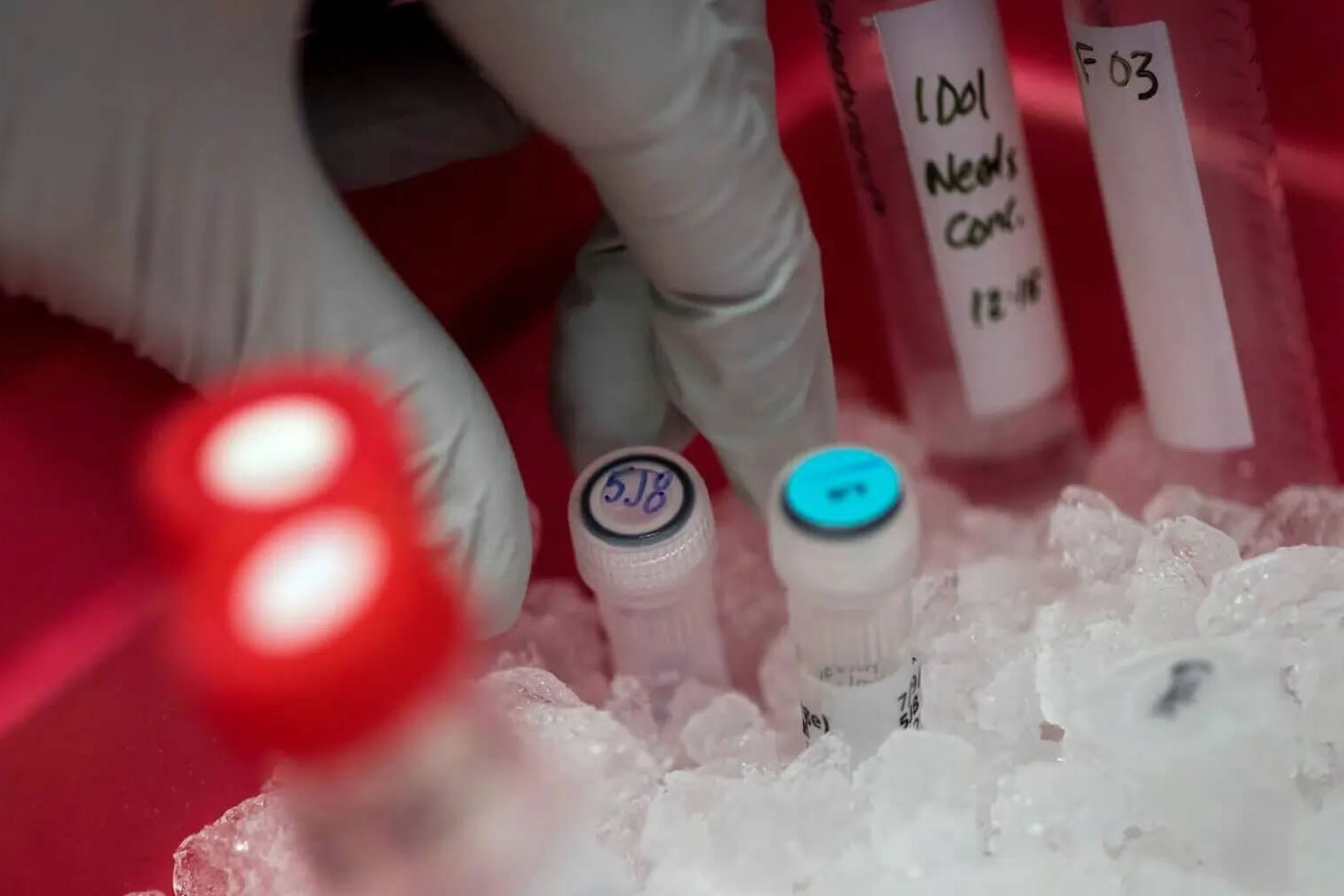
In the pharmaceutical industry, logistics isn’t just about moving goods from point A to B—it’s about safeguarding life-saving medicines, vaccines, and clinical trial materials. Every shipment carries responsibility, precision, and trust. At TFI, we understand the stakes, and we design our logistics solutions to meet the highest standards of care and compliance.
Whether you’re transporting temperature-sensitive vaccines, clinical trial specimens, or essential medicines, you can rely on us. Our trained and experienced team ensures that every shipment arrives safely, on time, and is fully compliant with Good Distribution Practice (GDP).
Inquire Now
Our Services
When it comes to pharmaceuticals and healthcare products, TFI’s services go beyond logistics. This is why we’ve built solutions that ensure your medicines, vaccines, and biological samples are handled with the highest standards of safety, compliance, and reliability. Whether it’s maintaining the cold chain, navigating customs, or providing specialized packaging, our focus is always on protecting product integrity and giving you confidence at every step of the journey.
Cold Chain Management
We maintain strict cold chain controls from pickup to delivery to preserve the integrity of pharmaceuticals and biological samples with 15–25°C, 2–8°C, and -20 to -80°C temperature requirements
Global Freight Forwarding

As a GDP-certified agent with 35+ years’ experience, we provide door-to-door delivery ensuring your time-sensitive shipments arrive on schedule
Specialized
Packaging
We provide WHO-approved passive packaging solutions that safeguard your pharmaceutical products while meeting strict regulatory standards
Regulatory Compliance
We take the stress out of compliance by handling all regulatory requirements, so your shipments always meet international standards
Customs Clearance
From customs paperwork to compliance checks, our in-house brokers seamlessly navigate international regulations and Emirates Drug Establishment (EDE) approvals so your time-critical cargo never faces delays
Qualified Transportation
Our pharma-dedicated fleet is temperature-mapped, temperature-controlled, and GPS-tracked, ensuring safe and compliant deliveries every time
GDP Standards We Live By
At TFI, Good Distribution Practices (GDP) aren’t just regulations we follow—they’re the foundation of how we move pharmaceuticals.
Temperature Control
We ensure every pharmaceutical product is stored and transported under the exact conditions it needs, safeguarding its quality and effectiveness from origin to destination.

Documentation & Traceability
We maintain accurate records of every handoff, storage condition, and movement.

Qualified Equipment & Vehicles
We take pride in our pharma-dedicated climate-controlled fleet, qualified cold-chain packaging, and real-time GPS & temperature monitoring systems.

Training &
Personnel
Our in-house team is GDP-trained in handling pharmaceutical products with strict protocols.

Route Optimization and Risk Management
We have robust contingency plans for delays, temperature excursions, or transport disruptions.


Why Choose TFI For
Healthcare Logistics?
Committed to the integrity and safety of every shipment, TFI specializes in handling sensitive pharmaceutical products with precision and reliability. As a GDP-certified company with GDP-trained personnel, we uphold the highest standards of compliance and quality at every step of your supply chain.
- GDP-compliant processes
- Emergency / same-day delivery options
- Pharma-dedicated, temperature-mapped vehicles
- Real-time temperature monitoring
- Real-time GPS tracking
- ISO-9001 and ISO-45001
- 35+ years' industry experience
- Licensed Dangerous Goods agent
- Passive packaging solutions
- Blood samples & human specimens
- Vaccines and biologics
- Clinical trial materials (investigational drugs, comparators, lab kits)
- Temperature-sensitive medicines (chilled, frozen, or ambient-controlled)
- Plasma and derivatives
- Cell and gene therapies (including stem cells and CAR-T)
- Oncology products
- Specialty medicines (for rare and chronic diseases)
- Pharmaceutical APIs (Active Pharmaceutical Ingredients)
- Medical devices & diagnostic kits
- Controlled substances (in compliance with UAE regulations)
- Over-the-counter (OTC) medicines
- Critical spare parts for medical equipment
Our Networks and Accreditations




Our Networks and Accreditations
About Us
At TFI, we specialize in pharmaceutical logistics designed to protect product integrity at every stage of the supply chain.
As a GDP-certified provider, we adhere to the highest standards in temperature control, regulatory compliance, and secure handling, ensuring that every shipment meets strict quality requirements. From comprehensive end-to-end cold chain management to timely global deliveries, our expertise covers medical equipment, clinical trial specimens, biologics, and other sensitive healthcare products.
By combining advanced technology, real-time monitoring, and a dedicated team of GDP-trained professionals, we guarantee that your critical shipments reach their destinations safely, efficiently, and on schedule, giving you complete peace of mind.

ISO 9001:2015
ISO 45001: 2018
www.tuv.com
ID: 9000012225